By What Means Can The Internal Energy Of A Closed System Increase?
By what means can the internal energy of a closed system increase?. For a closed system no mass transfer process proceeding between two states. And for most of what were going to do you can assume that were dealing with an ideal gas. Being a state function means that E has the following property.
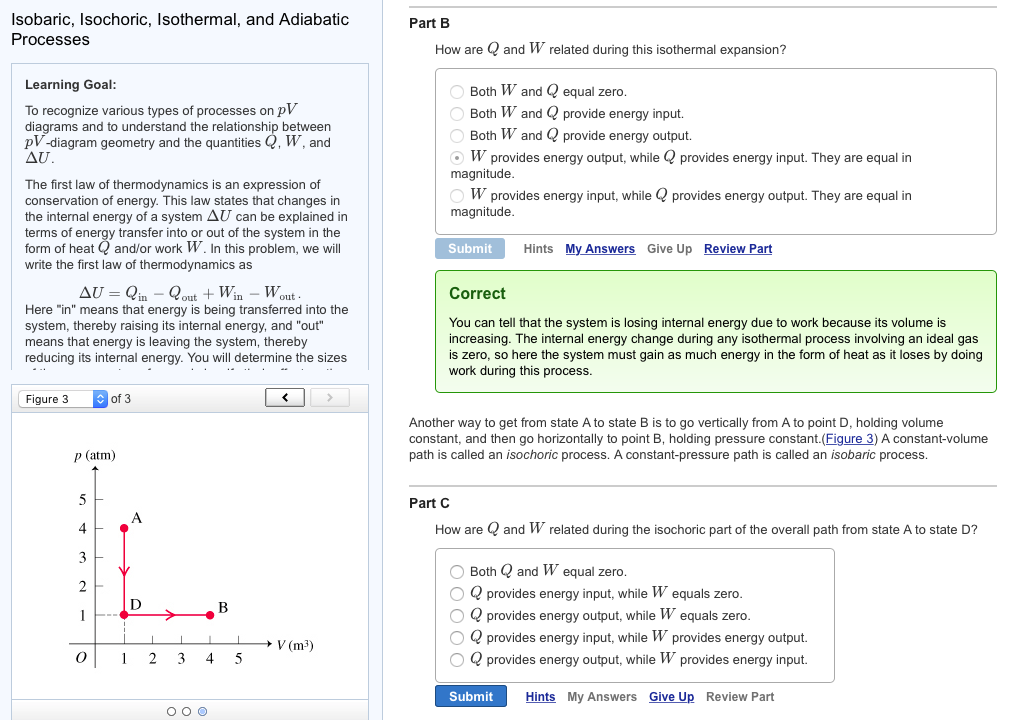
1 it is a state function and 2 it scales as an extensive. A closed system can exchange energy with its surroundings through heat and work transfer. Any heat interaction that takes place in the system with its surroundings also changes its internal energy.
Internal Energy E measures the energy state of a system as it undergoes chemical andor physical processes. Now we also said in the last video that because internal energy is all the energy in the system it cant be randomly created or destroyed. Internal Energy Work Heat and Enthalpy 15 More general formula for PV work P does not need to be constant f i V V ext w P dV ³ Sign Convention.
When there are energy transfers in a closed system there is no net change in the total. When the system increases its general velocity. But since energy remains constant from the first law of thermodynamics the total change in internal energy is always zero.
Instead of it becomes a lot more complicated with solids and conductivity and vibrations and all that. A closed system falls in between an open system and an isolated system in terms of outside influence. A higher internal energy means a higher temperature.
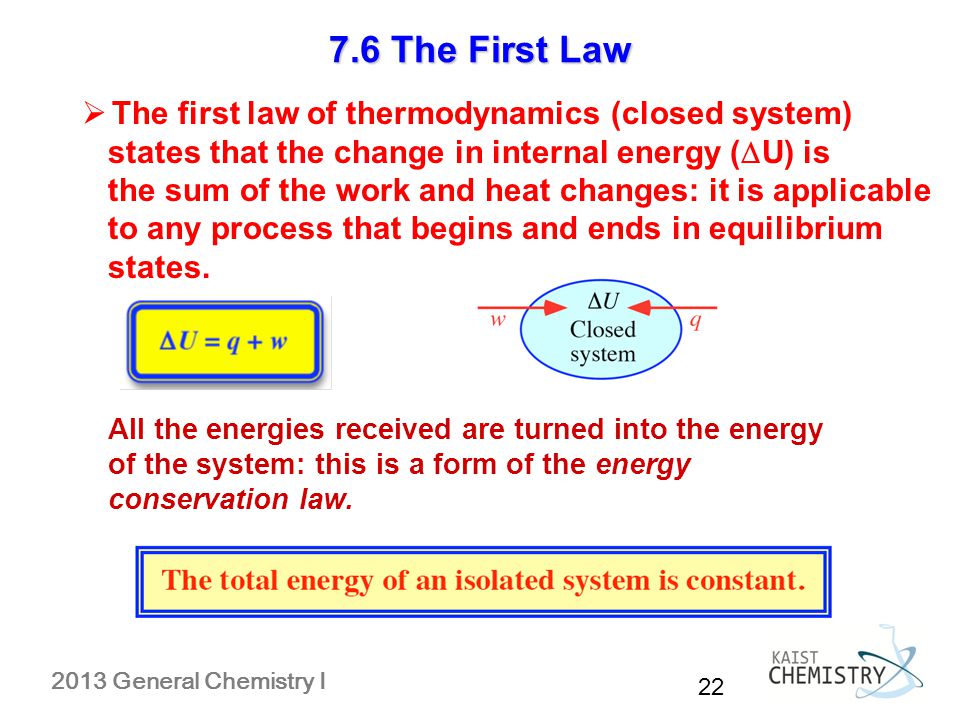
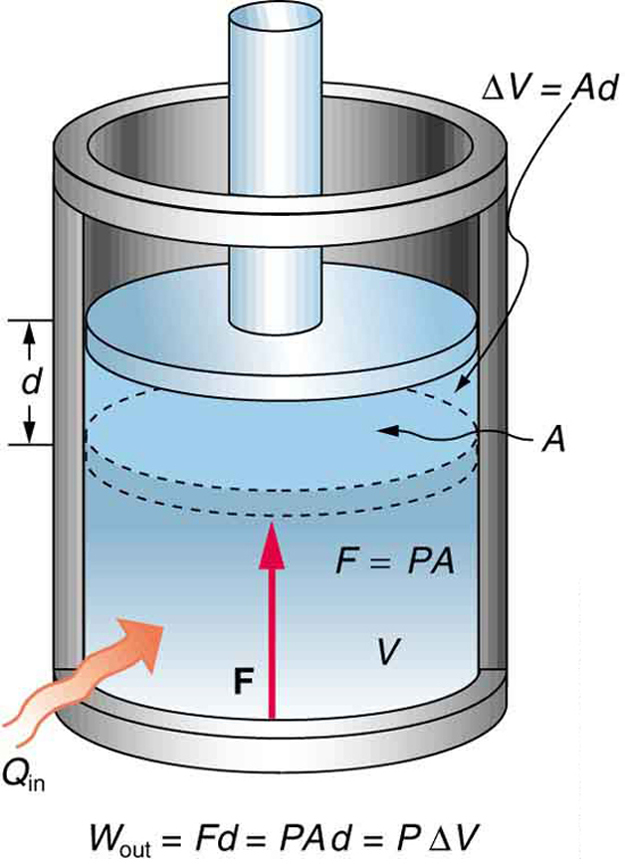
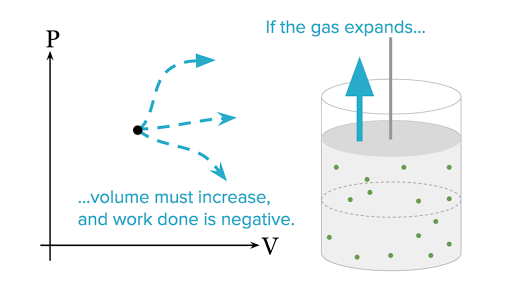
ΔU Q W where ΔU denotes the change of the systems internal energy Q the quantity of energy added to it as heat and W the work done by the system on its surroundings. 0 Work done by the system lowers the internal energy w 0 Other forms of work. If work is done by the system positive W its internal energy decreases.
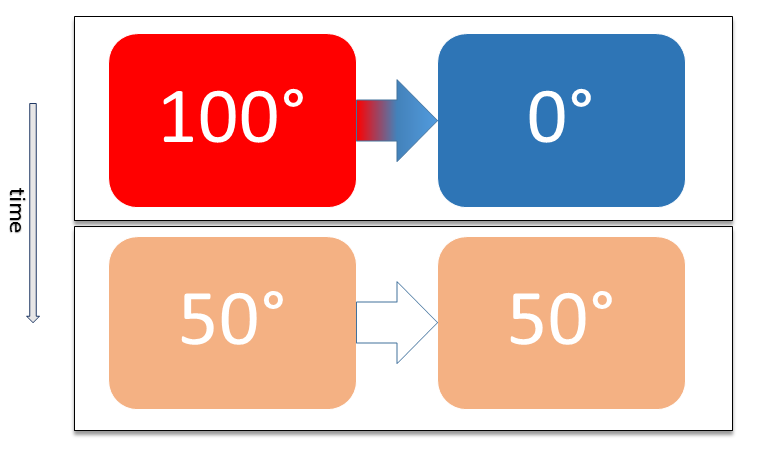
When a closed system receives energy as heat this energy increases the internal energy. Based on kinetic theory heat is defined as the energy associated with the random.
The gas expands from initial volume of 0001 m 3 and simultaneously the external load of the piston slowly and continuously decreases from 1 MPa to 05 MPa.
As explained above Cp is always greater than Cv because the amount of heat supplied at constant pressure is utilized in two ways 1 increasing the internal energy and hence temperature since. It can just be transformed from one form to another. The internal energy would increase if work is done on the system and decreases if work is done by the system. Thermodynamics Unit Internal Energy Work and Heat 1. ΔE ΔKEΔP E ΔU Q W. Assume an isothermal expansion of helium i f in a frictionless piston closed system. Internal Energy E measures the energy state of a system as it undergoes chemical andor physical processes. But since energy remains constant from the first law of thermodynamics the total change in internal energy is always zero. Any heat interaction that takes place in the system with its surroundings also changes its internal energy.
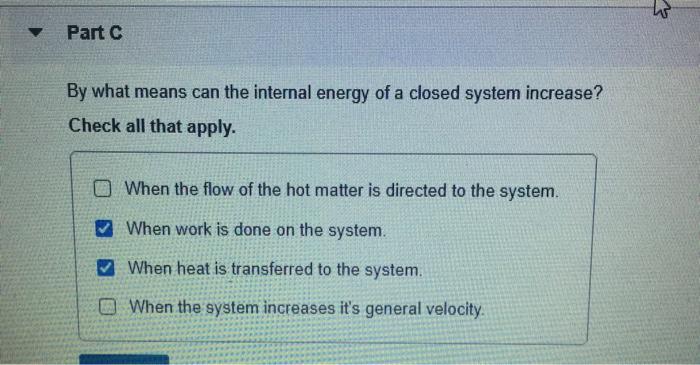
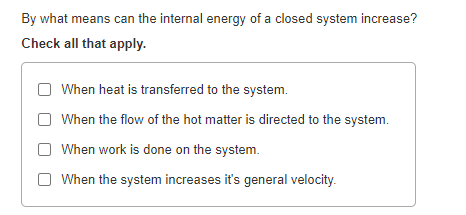
Note the value of heat and work as they are transferred into or outof a system. Work done on the system raises internal energy of system w. When a closed system receives energy as heat this energy increases the internal energy. Any heat interaction that takes place in the system with its surroundings also changes its internal energy. Q 0 q negative heatlost. The internal energy of a system increased by 982 J when it absorbed 492 J of heat. When heat is transferred to the system when work is done on the system when the flow of the hot matter is directed to the system.




























Post a Comment for "By What Means Can The Internal Energy Of A Closed System Increase?"